Silexion Therapeutics Reports Positive Preclinical Data Demonstrating SIL204’s Reach and Activity in Major Pancreatic Cancer Metastatic Sites Following Systemic Administration
New Positive Preclinical Data Shows Succesful Drug Distribution to Liver, Peritoneum, and Lung with Measurable Reductions in Tumor Burden at Clinically Relevant Doses
Results Provide Further Validation of the Systemic Component of a Dual-Route Administration Strategy Enabeling Potential Targeting of Both Primary Tumors and Metastatic Disease
Silexion Remains on Track for Phase 2/3 Trial Initiation in H1 2026 Following Planned Q4 2025 and Q1 2026 Regulatory Submissions
GRAND CAYMAN, Cayman Islands, Sept. 11, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced new preclinical data demonstrating that subcutaneously administered SIL204 successfully reaches all primary sites of pancreatic cancer metastasis and shows anti-tumor activity.
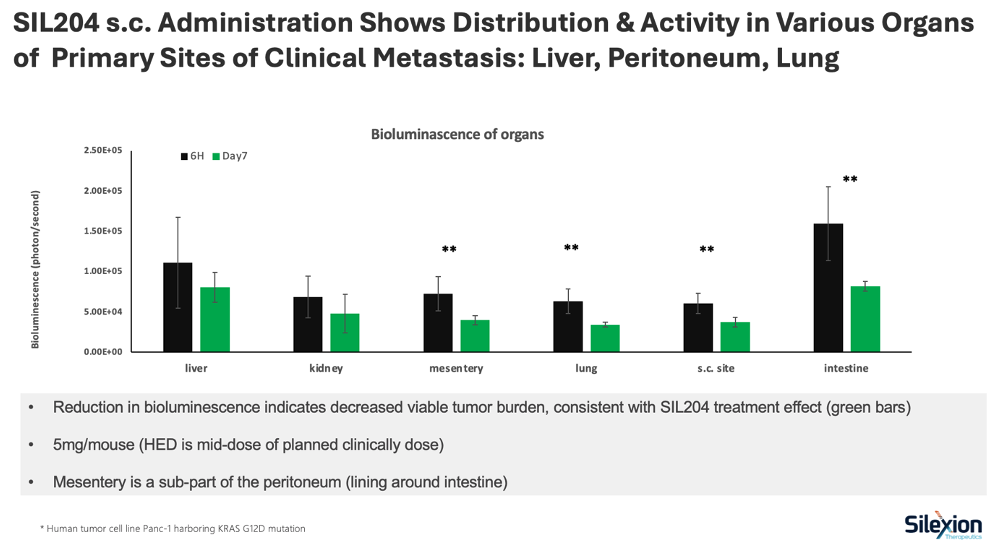
The study evaluated SIL204's biodistribution and therapeutic activity following subcutaneous administration in a metastatic pancreatic cancer mouse model using bioluminescent imaging. Results confirmed that SIL204 distributed to key organs where pancreatic cancer commonly spreads, with measurable reductions in tumor burden observed across multiple sites.
The ability to reach metastatic sites is particularly important given that over 80% of pancreatic cancer mortality is attributed to metastatic disease, and more than 40% of initially resectable patients experience recurrence within 12 months, predominantly as distant metastases.
"These findings provide additional validation for a critical component of our dual-route administration strategy - the ability of subcutaneously delivered SIL204 to reach metastatic sites throughout the body," said Mitchell Shirvan, Ph.D., Chief Scientific Officer of Silexion. "Demonstrating drug distribution to the liver, peritoneum, and lung, which represent the primary sites of metastatic pancreatic cancer spread, supports our approach of combining intratumoral delivery for primary tumors with systemic administration for disseminated disease."
Key Study Findings:
- SIL204 successfully distributed to all major metastatic sites following a single subcutaneous injection at 5mg/mouse (mid-range human equivalent dose for planned clinical trials)
- Reductions in bioluminescent signal, indicating decreased tumor burden, were observed at day 7 across all evaluated organs
- Statistically significant reductions (p<0.01) were achieved in the peritoneum (mesentery), lung, and intestine
- The liver, the most common site of pancreatic cancer metastasis, showed measurable reduction in tumor burden
- Studies utilized human pancreatic cancer cells (Panc-1) harboring the KRAS G12D mutation
- The use of human equivalent dosing demonstrates that these results were achieved at drug concentrations directly relevant to planned clinical use, providing important validation for the transition from preclinical to human studies
"This data addresses a fundamental challenge in pancreatic cancer treatment - reaching micrometastases that have spread beyond the primary tumor," added Ilan Hadar, Chairman and CEO of Silexion. "Combined with our previously reported intratumoral efficacy data, we now have evidence supporting both components of our treatment approach designed to comprehensively address this aggressive disease."
The Company is also conducting expanded tissue culture studies across multiple cancer types and KRAS mutations to further characterize SIL204's pan-KRAS potential, with results expected in the near future.
Silexion remains on track to initiate Phase 2/3 clinical trials evaluating its dual-route administration approach in the first half of 2026, with regulatory submissions planned for Q4 2025 and Q1 2026.
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical stage, oncology-focused biotechnology company dedicated to the development of innovative treatments for unsatisfactorily treated solid tumor cancers which have the mutated KRAS oncogene, generally considered to be the most common oncogenic gene driver in human cancers. The Company conducted a Phase 2a clinical trial in its first-generation product which showed a positive trend in comparison to the control of chemotherapy alone. Silexion is committed to pushing the boundaries of therapeutic advancements in the field of oncology, and further developing its lead product candidate for locally advanced pancreatic cancer. For more information please visit: https://silexion.com
Notice Regarding Forward-Looking Statements:
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion's business strategy, preclinical and clinical development plans, timeline regulatory submissions and Phase 2/3 trial initiation, and expectations regarding SIL204's therapeutic potential, are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied in those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion's ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion's strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion's future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexion's ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed with the SEC by the Company, including the Company's Annual Report on Form 10-K for the year ended December 31, 2024. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact:
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
mirit@silexion.com
Capital Markets & IR Contact:
Arx Capital Markets
North American Equities Desk
silexion@arxadvisory.com

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

